Who Else Wants Info About How To Increase Boiling Point
Boiling points can be changed in several ways.
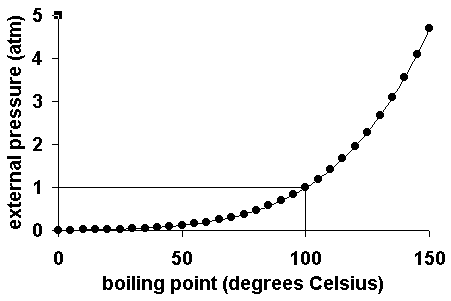
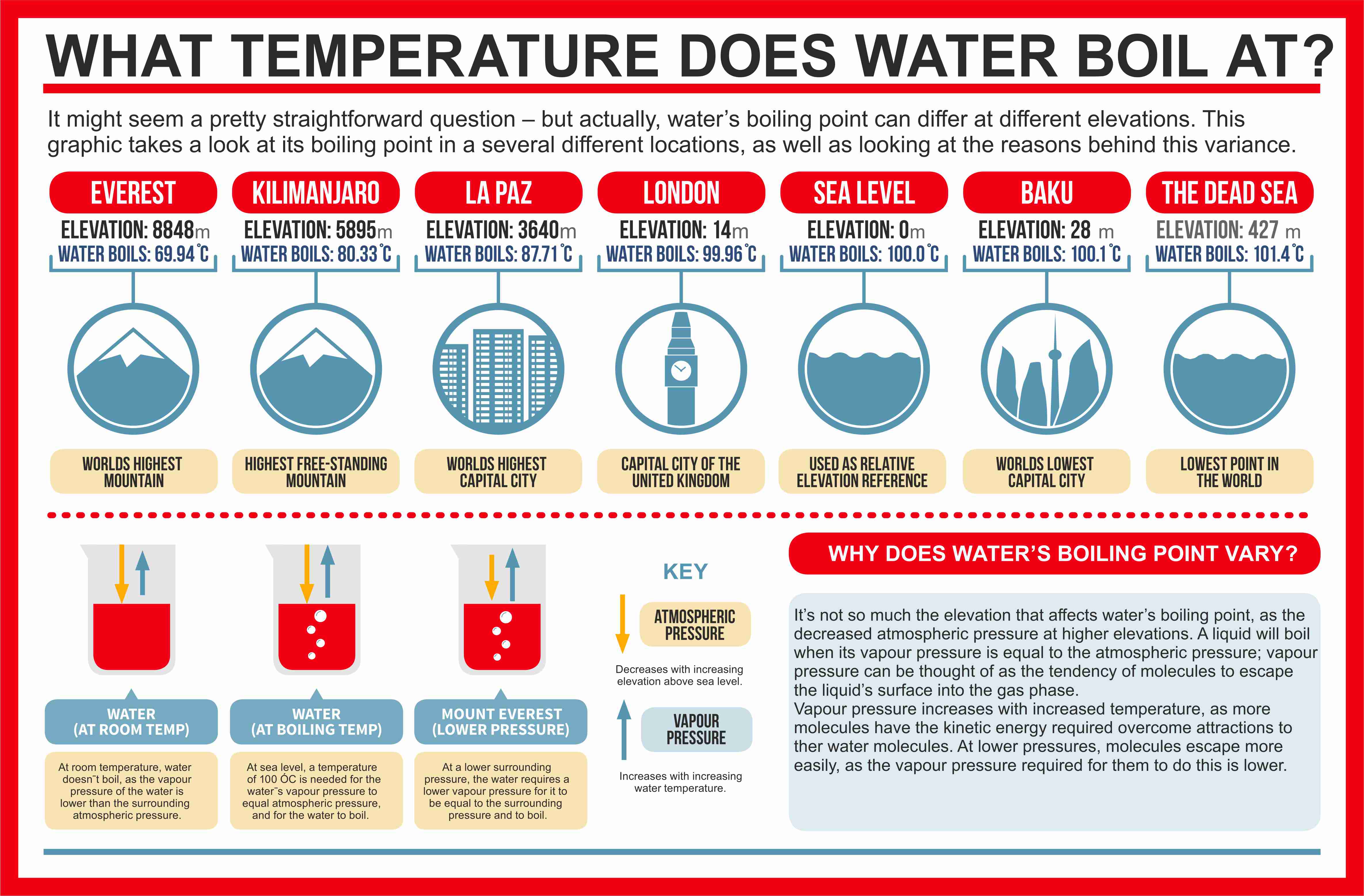
How to increase boiling point. The more they stick together, the more energy it will take to blast them. The output temperature is given as °c, °f, k and °r. If the pressure in the classroom is close to 1 atmosphere, then the.
So yes, salt increases the boiling temperature level, but not by quite. What are 2 ways to increase the boiling point? Water will boil at temperatures greater than 100℃ when:
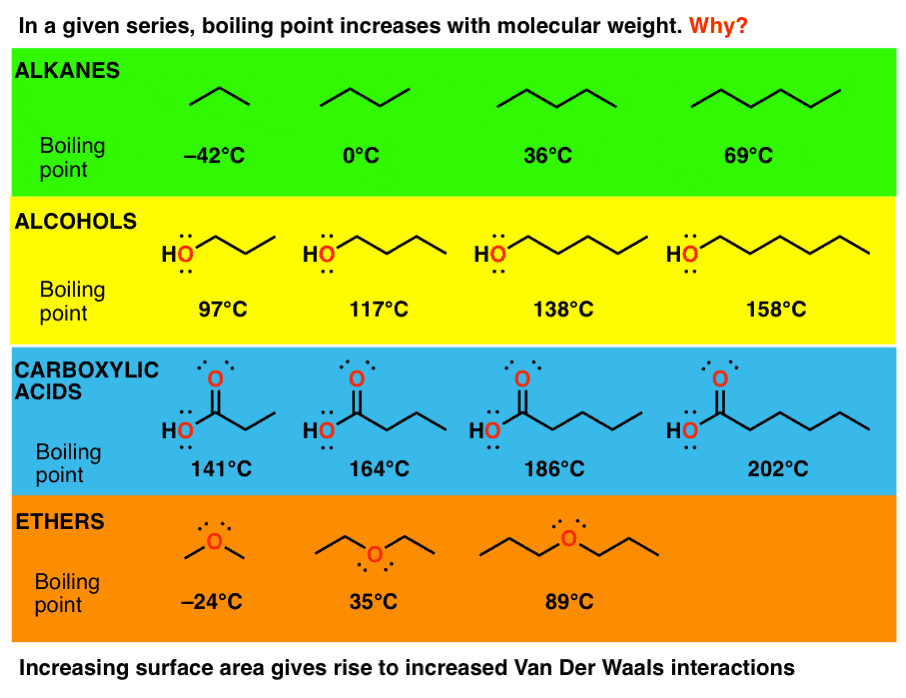
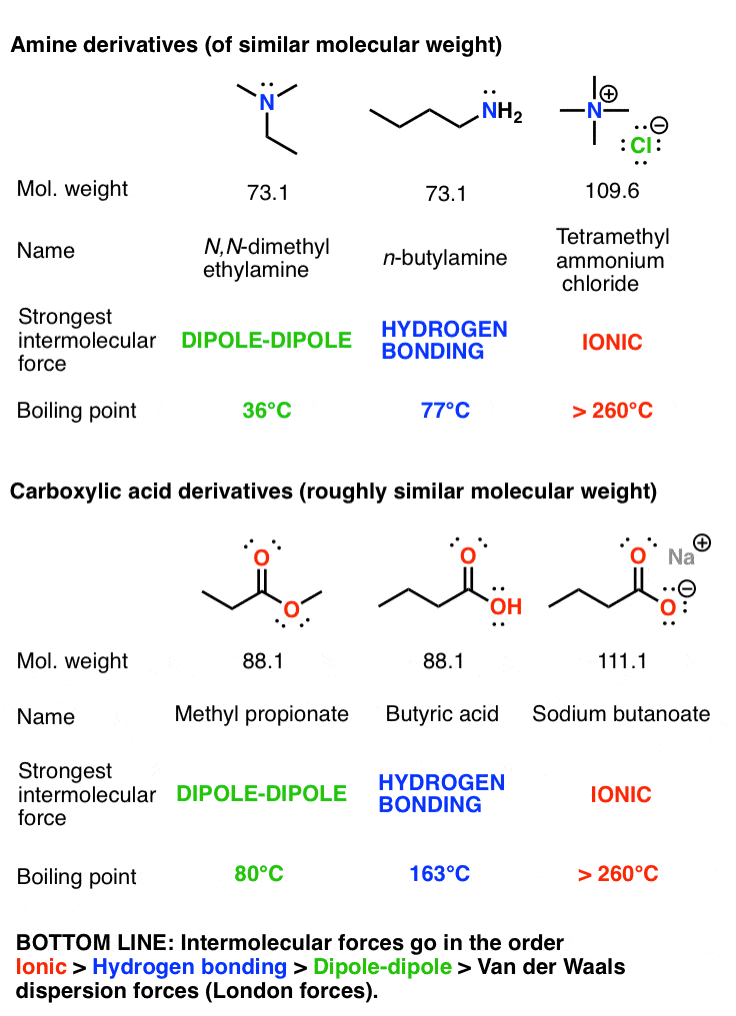
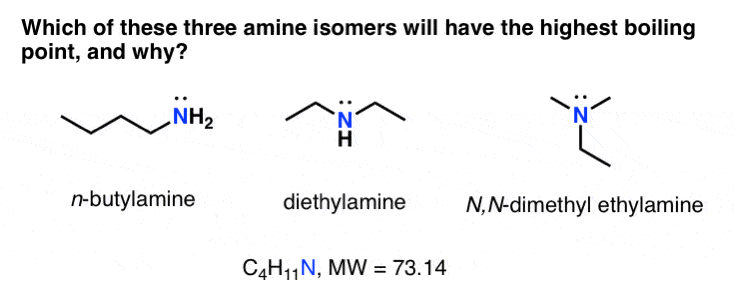
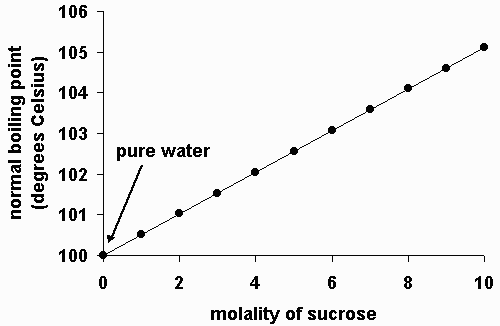
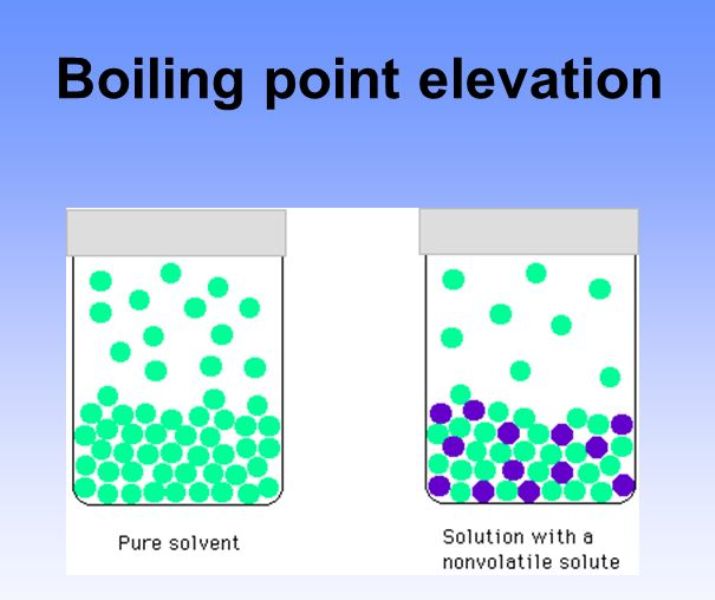
Boiling point elevation occurs when the boiling point of a solution becomes higher than the boiling point of a pure solvent. When an impurity is added to a liquid, its vapour pressure is likely to decrease, thereby increasing its boiling point. The more intermolecular mass is added, the higher the boiling point.
The addition of solutes or other substances usually changes the boiling point. If you add 20 grams of salt to five litres of water, instead of boiling at 100° c, it’ll boil at 100.04° c. This increase results in the liquid boiling at a higher temperature the experiment will test the following hypothesis:
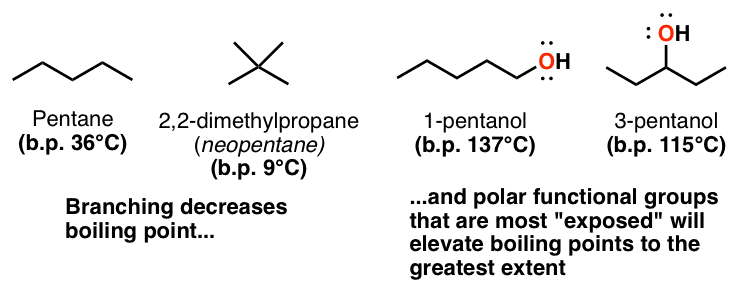
The temperature at which the solvent boils is increased. The key thing to consider here is that boiling points reflect the strength of forces between molecules. This protects the system from boiling over at the top of the operating temperature range.
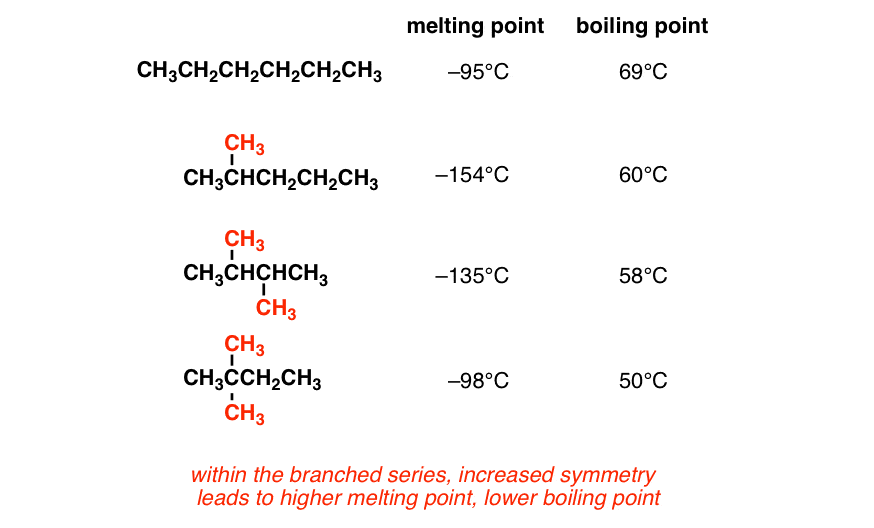
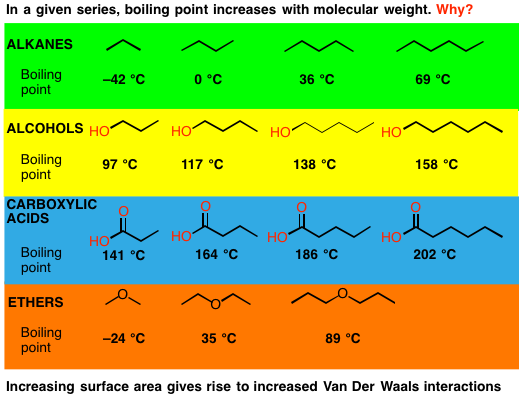
Boiling points of alkenes depends on more molecular mass (chain length). The addition of solutes or other substances usually changes the boiling point. A 2.5 pound per square inch pressure raises the boiling point up to.